UpToDate on X: "The @US_FDA has approved Zelsuvmi (berdazimer) topical gel to treat molluscum contagiosum in adults and pediatric patients 1 year and older. Zelsuvmi is expected to launch in the second
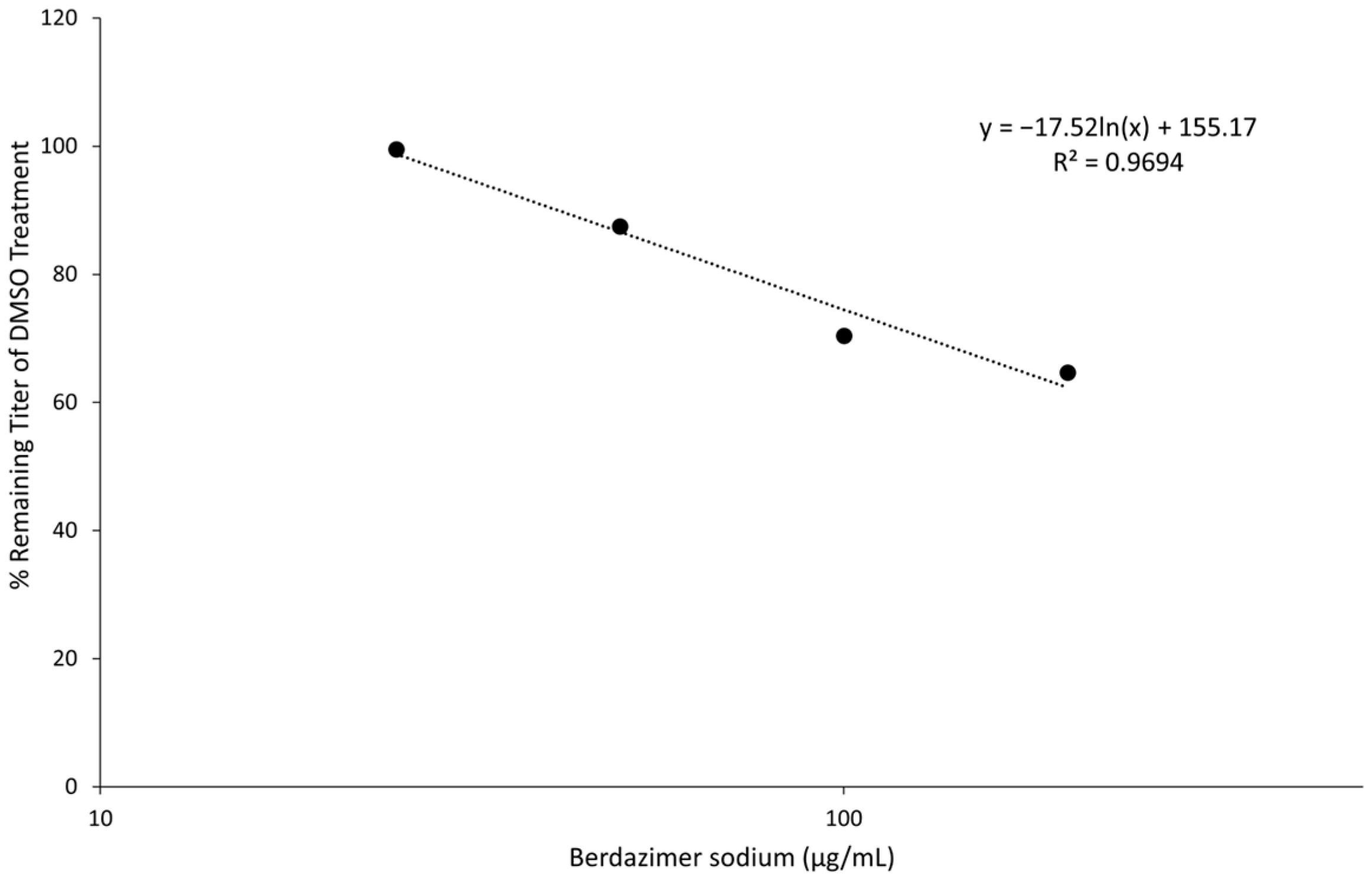
Viruses | Free Full-Text | The Antiviral Effect of Berdazimer Sodium on Molluscum Contagiosum Virus Using a Novel In Vitro Methodology
Berdazimer 10.3% Gel, a Nitric Oxide-Releasing Topical Medication for Molluscum Contagiosum, Triggers BOTE (Beginning Of The End
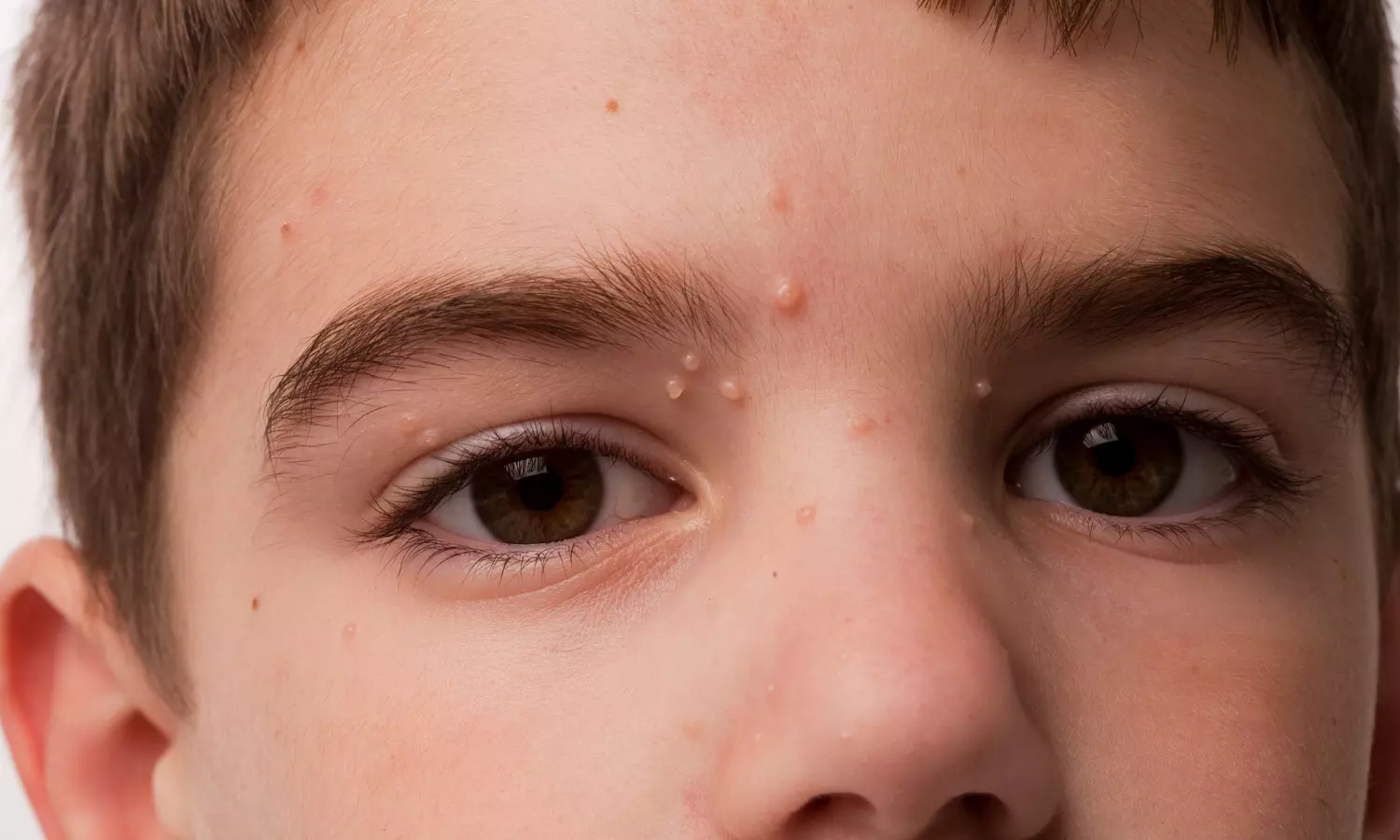
Berdazimer gel 10.3 % found effective in clearing molluscum lesions in phase 3 SIMPLE clinical trial
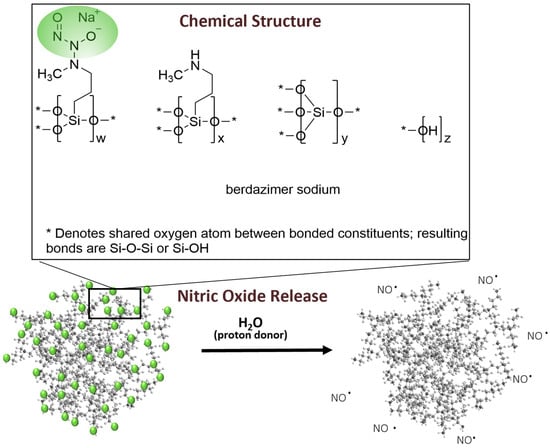
Viruses | Free Full-Text | The Antiviral Effect of Berdazimer Sodium on Molluscum Contagiosum Virus Using a Novel In Vitro Methodology

Arun C Inamadar MD , FRCP (Edin), FRCP(London) on X: "Recent update on treatment option : Use of berdazimer gel, 10.3%, for MC appears to demonstrate favorable efficacy and safety with low
Efficacy and Safety of Topical Nitric Oxide−Releasing Berdazimer Gel in Patients With Molluscum Contagiosum: Results from B-S
Efficacy and Safety of Topical Nitric Oxide−Releasing Berdazimer Gel in Patients With Molluscum Contagiosum: Results from B-S

Dermatology Times على LinkedIn: Novan Submits NDA for Berdazimer Gel for the Treatment of Molluscum…

Pharmacokinetic Profile, Safety, and Tolerability of Topical Berdazimer Gel, 10.3% in Patients With Molluscum Contagiosum - JDDonline - Journal of Drugs in Dermatology

Novan on X: "FDA Accepts Novan's NDA for Berdazimer Gel, 10.3% for the Treatment of Molluscum Contagiosum with a PDUFA Goal Date of January 5, 2024. https://t.co/oSLxQBEnP5 $NOVN #dermatology https://t.co/R8htaI7pgM" / X